Dangane da samfurin atomic na Niels Bohr, protons da neutrons na atom suna cikin tsakiya, yayin da electrons ke kewaye da shi. Kodayake ba za mu iya sanin inda lantarki yake daidai ba, akwai wuraren da ake iya samun sa, atomic orbitals. Kuma ta yaya zamu iya tantance waɗancan orbitals? Mai sauqi, ta amfani da lambobin jimla.
Menene lambobin jimla?
Akwai lambobin jimla 4. Uku daga cikinsu suna ba mu bayanai game da inda wani electron na wani atom yake, wato, suna ba mu bayanai game da ƙwanƙwasa. A gefe guda, lambar jimla ta huɗu ba ta gaya mana inda wutar lantarki take ba, amma ta yaya. Har yanzu ba ku da cikakken bayani game da wannan? Ku tafi!
- Babban lambar adadi (n). Ita ce matakin kuzari na ƙarshe don cikawa kuma yana nuna girman ƙwanƙwasawa don haka nisan tsakanin nucleus da electron. Me ya sa? Mai sauqi. Girman maɗaukaki, yadda electron zai iya kasancewa daga tsakiya na atom.
- Azimuthal ko lambar adadi na biyu (l). Nuna siffar orbital.
- Lambar jimlar Magnetic (m). Yana nuna daidaituwa na orbital.
- Lambar adadi (s). Faɗa wace hanya ce lantarki ke juyawa.
Sauƙi daidai? Bari mu tafi tare da muhimmin abu!
Ta yaya ake samun adadi mai yawa
Don samun lambobin jimla kawai dole ku bi matakai 2 masu sauƙi:
- Rubuta tsarin lantarki.
- Sami lambobi masu ƙima daga nau'in lantarki daban (na ƙarshe wanda ke cika orbital).
Tsarin lantarki
Mun fara da mataki na 1, rubuta tsarin lantarki. yaya? Akwai hanyoyi guda biyu don yin ta, bari mu kai gare ta!
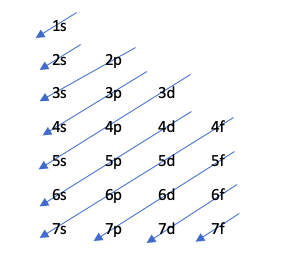
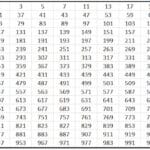
Tsarin Moeller
Wannan dabarar tana nuna odar cika abubuwan orbitals ta hanyar zane mai zuwa:
Wannan jadawalin yana ƙarƙashin ƙa'idar Aufbau, wanda ke kare cewa maƙallan sun cika girma da ƙarfi na tsarin makamashi, wato, kumburin da ke da mafi ƙarancin kuzari zai cika a baya.
Don gano wane orbital yana da ƙarin ƙarfi, ana yin aikin n + l. Idan wannan aiki na atom biyu daban ya haifar da lamba ɗaya, wanda adadin n ya fi girma zai sami ƙarin kuzari. A takaice, idan akwai taye, wanda ke da mafi ƙarancin lamba n an cika shi da farko. Bari mu gani tare da misali:
4p: n + l -> 4 + 1 = 5
5s: n + l -> 5 + 0 = 5
Tunda akwai taye a cikin dokar n + l, ya cika 4p a baya saboda lambar n ta ƙasa.
Kernel Model
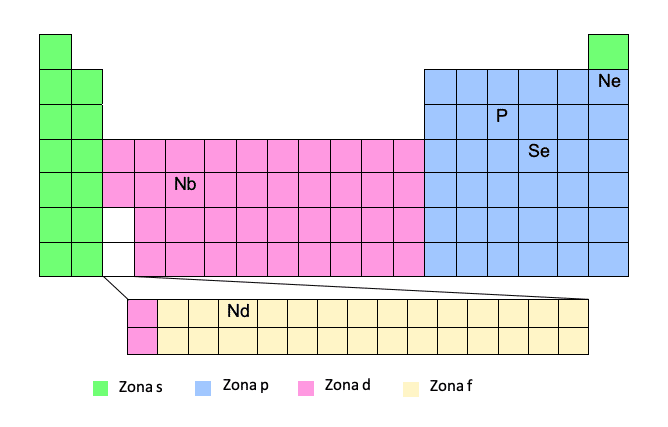
Don samun saitin lantarki bayan wannan ƙirar dole ne ku san teburin lokaci -lokaci sosai. Idan muna da lambar atomic da matsayin abun cikin tebur, yanki ne na waina!
Ana ɗaukar wannan hanyar a matsayin hanya mafi sauƙi tunda yana ba da damar rashin rubuta cikakkiyar daidaiton lantarki. Ta wannan hanyar, zamu iya rubuta sunan iskar gas mai daraja a sama a cikin brackets, sannan hanyar daga wannan gas ɗin mai daraja zuwa sinadarin da ake tambaya. Bari mu ga misali:Don haka, za mu rubuta yanayin ta la'akari da adadin lokacin (jere na teburin lokaci) da "yankin" kuma, da zarar an rubuta tsarin lantarki, za mu fitar da adadi mai yawa.
Za a rubuta phosphor (P) daga gas mai daraja ta baya, wato, Neon:
P -> [Ne] 3s23p3
Tabbas, dole ne kuyi taka tsantsan da wannan hanyar, tunda yankuna d da f sune yankuna na musamman. Yayin da muke tafiya, a zone d ba za mu sanya adadin lokacin (jere) ba, amma adadin lokacin ya rage ɗaya. Haka yake faruwa da yankin F, ba za mu saka adadin lokacin ba, amma adadin lokacin ya rage biyu. Za ku fahimce shi da kyau tare da misalai guda biyu:
Nb -> [Kr] 5s14d4
Kodayake yana cikin lokaci na 5, lokacin da muke cikin zone d, mun cire 1.
Nd -> [Xe] 6s24f14
Kodayake yana cikin lokaci na 6, lokacin da muke cikin yankin f, mun cire 2.
Banbanci a cikin tsarin lantarki
Tsarin lantarki yana da wasu fannoni na musamman waɗanda, idan ba ku san su ba, na iya haifar da manyan masu ciyar da kai. Amma kar a yada tsoro! Za mu gaya muku!
Yankin F
Yankin F yana bayyana a kasan teburin lokaci -lokaci, amma a zahiri "an saka" a cikin rata da muke gani cikin farin, wato tsakanin abubuwa na farko da na biyu na layuka biyu na ƙarshe na yankin D.
Kuna gani? A saboda wannan dalili, wani lokacin, lokacin da zamu rubuta tsarin lantarki na wani kashi a yankin F, alal misali, Nd, dole ne mu sanya lantarki a sashi na D na matakin da ya dace dangane da wannan ɓangaren a yankin D wato kafin shiga yankin F.
Ce -> [Xe] 6s25d14f1
Rukuni na 6 da Rukuni na 11
Rukuni na 6 da rukunin ƙarfe na 11 suna da electrons 4 da 9 a cikin bawo na ƙarshe, bi da bi. Sabili da haka, don zama mafi daidaituwa, s orbital yana farin ciki kuma yana rasa lantarki, wanda ke wucewa zuwa gaba mai zuwa, d. Ta wannan hanyar, za a bar s orbital tare da lantarki; da d tare da 5, idan ya kasance wani ɓangaren rukuni na 6, ko tare da 10, idan ɓangaren ƙungiyar 11 ne.
Ga misali:
Ag -> [Kr] 5s24d9
A bayyane yake, wannan zai zama tsarin lantarki na azurfa (Ag). Koyaya, rasa lantarki daga s orbital, yana kama da wannan:
Ag -> [Kr] 5s14d10
Koyaya, akwai keɓaɓɓu ga wannan ƙa'idar, kamar Tungsten (rukuni na 6), wanda aka bari tare da 2 electrons a cikin s orbital da 4 a d orbital.
Amma kada ku damu! Mafi na kowa (Cr, Cu, Ag da Au) suna bin wannan doka.
Kuna samun shi? To. Wannan shine abin da kuke buƙatar sani game da daidaitawar lantarki. Bari mu tafi don lambobi masu yawa!
Yadda ake samun adadi mai yawa
Domin samun lambobi masu ƙima, dole ne mu san adadin electrons ɗin da suka dace a cikin kowane harsashin orbital, la'akari da cewa electrons 2 sun dace a cikin orbital.
- Layer s. Yana da madaidaiciyar madaidaiciya guda ɗaya, don haka yana iya ɗaukar electrons 2.
- Layer p. Yana da 3 orbitals, don haka akwai dakin 6 electrons.
- Layer d. Yana da 5 orbitals, don haka yana iya dacewa da electrons 10.
- Layer f. Yana da 7 orbitals, wato yana riƙe da electrons 14.
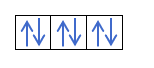
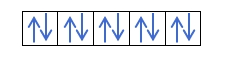
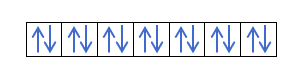
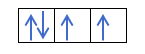
Yanzu da kuka fahimci cewa akwai electrons 2 a cikin kowane kewaya, yakamata ku san mulkin Hund. Wannan ƙa'idar ta ce lokacin da ake cika madaidaiciyar madaidaiciyar madaidaiciya ko harsashi, alal misali, harsashin p, electrons ɗin suna cika keɓaɓɓiyar hanya ɗaya (tabbatacce) sannan a ɗayan (mara kyau). Kuna son ganin ta da misali?
Idan muna da 2p4, wato, 2p orbital tare da electrons 4, ba zai cika kamar haka ba:
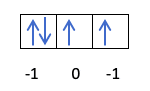
Zai cika kamar haka:
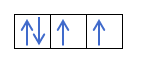
Kuna samun shi? Mai girma, bari mu ga yadda ake lissafin lambobi:
- Yawan adadi n. Wannan lambar ta zo daidai da adadin matakin ƙarshe na daidaitawar lantarki. Misali, idan tsarin lantarki ya ƙare a cikin 4s2, babban adadin adadi zai zama 4.
- Yawan adadi l. Wannan adadi ya dogara da Layer na ƙarshe da aka cika.
- Layer s -> l = 0
- Layer p -> l = 1
- Layer d -> l = 2
- Layer f -> l = 3
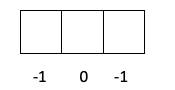
- Yawan adadi m. Lambar m na iya zama kowane ƙima tsakanin -l zuwa + l, don haka zai dogara ne akan ƙaramin abin hawa wanda bambancin lantarki yake ciki, wato idan yana da s, p, d ko f. Yadda ake lissafin wannan lambar ya fi rikitarwa, bari mu gan ta tare da zane biyu:
- Layer s -> Kamar yadda muka gani, l yana da darajar 0, don haka m zai iya zama darajar 0 kawai.
- Layer p -> L yana da daraja 1, don haka m na iya zama -1, 0 ko 1.
- Layer d -> L shine 2, don haka m na iya zama -2, -1, 0, 1 da 2.
- Layer f -> l yana da daraja 3, don haka m na iya zama -3, -2, -1, 0, 1, 2 da 3.
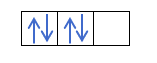
Kun rigaya kun san yadda ake cika kewayawa, don haka adadin adadi na m zai sami ƙimar ramin inda electron da aka zana na ƙarshe yake. Kuna tuna wannan misalin daga baya?:
A wannan yanayin, m zai kasance -1, tunda a cikin kwalin p (3 orbitals), idan akwai electrons 4, na ƙarshe da za a cika zai zama mummunan na farkon orbital.
- Yawan adadi s. Adadin lamba s zai iya zama darajar ½ da -½ kawai. Idan electron na ƙarshe da aka zana yana da kyau, wato, kibiya ta tashi, s zai zama ½. A gefe guda kuma, idan electron na ƙarshe da ya cika maɗaukaki mara kyau, wato, tare da kibiya yana nuna ƙasa, s zai kasance -½.
Ayyuka da misalai
Ee, mun riga mun san cewa duk wannan bayanai ne masu yawa, amma za ku fahimce shi da kyau tare da wasu misalai. Mu je zuwa!
Misali 1
Selenium (Se) -> Lambar Atomic: 34
- Muna rubuta tsarin lantarki. Muna rubuta daidaiton wutar lantarki bisa ga tsarin Moeller, la'akari da cewa s, p, d da f orbitals suna da 2, 6, 10 da 14 electrons bi da bi. Muna rubuta saitin ta ƙara adadin electrons, wanda aka rubuta azaman mai faɗaɗawa.
1s22s22p63s23p64s23d104p4
Kamar yadda 4p orbital bai cika ba, tunda electrons zai ƙara har zuwa 36, ba mu sanya 4p ba6amma 4p4.
- Muna fitar da lambobi masu yawa. Don yin wannan, muna duban valence ko electron mai banbanci, wato, electron na ƙarshe wanda ya cika kumburin. A wannan yanayin, zamu kalli 4p4.
- Babban lambar adadi. Matsayin makamashi na ƙarshe don cika shine 4.
n = 4
- Lambar adadi na sakandare. Ƙarshen ƙarfin kuzari na ƙarshe don cika shine p orbital.
l = 1
- Lambar jimlar Magnetic. Idan muna zana masu zaɓin lantarki, na ƙarshe da za a cika shi ne farkon kewayawar harsashin p.
m = -1
- Lambar adadi. Wutar lantarki ta ƙarshe da za ta mamaye sararin samaniya tana da kibiyar ƙasa.
s = -½
Misali 2
Zinariya (Au) -> [Xe] 6s14f145d10
- Babban lambar adadi -> n = 5
- Lambar adadi na biyu -> l = 2
- Lambar jimlar Magnetic -> m = 2
- Lambar adadi mai juyi -> s = -½
Kuma shi ke nan! Yanzu lokaci ya yi da ku, za ku iya yin tsarin lantarki kuma ku sami lambobi masu yawa na abubuwa masu zuwa:
Cr (24), Rb (37), Br (35), Lu (71), Au (79)