Zvinoenderana nemhando yeatomu yaNiels Bohr, ma proton nema neutron eatomu ari mukakasi, nepo maerekitironi ari paari. Kunyangwe isu tisingakwanise kuziva chaizvo kuti elektroni iripi, pane nzvimbo dzingangowanikwa, maatomic orbitals. Uye isu tingaone sei iwo maokitori? Yakapusa kwazvo, uchishandisa huwandu hwehuwandu.
Ndeapi manhamba ehuwandu?
Pane mazana manhamba. Vatatu vavo vanotipa ruzivo nezve iko elektroni yeimwe atomu iri, ndiko kuti, ivo vanotipa ruzivo nezve iyo orbital. Kune rimwe divi, iyo yechina huwandu hwehuwandu hautiudze iko elekitironi, asi sei. Iwe hausati wanyatsojeka nezve izvi? Zvitore!
- Yakakura nhamba yehuwandu (n). Ndiyo yekupedzisira simba rezinga rekuzadza uye rinoratidza saizi yeiyo orbital uye nekudaro nhambwe iri pakati penyukireoni neelectron. Sei? Zviri nyore kwazvo. Iyo yakakura iyo orbital, iyo inowedzera iyo elektroni inogona kubva kunucleus yeatomu.
- Azimuthal kana yechipiri nhamba yehuwandu (l). Ratidza chimiro chemuchadenga.
- Nhamba yemagineti yakawanda (m). Inoratidza maitiro eiyo orbital.
- Spin yakawanda nhamba (s). Taurira nzira iyo electron inotenderera.
Nyore here? Ngatiendei nechakakosha chinhu!
Nhamba dzekuwanda dzakatorwa sei
Kuti uwane huwandu hwenhamba iwe unofanirwa kungotevera 2 matanho akareruka:
- Nyora iyo yemagetsi gadziriso.
- Tora huwandu hwehuwandu kubva pamusiyano elektroni (yekupedzisira inozadza iyo orbital).
Kugadziriswa kwemagetsi
Isu tinotanga nhanho 1, nyora iyo electron yekumisikidza. Sei? Pane nzira mbiri dzekuzviita, ngatisvikei pairi!
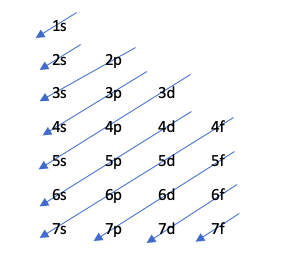
Moeller dhayagiramu
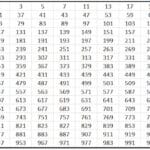
Iyi nzira inoratidza kurongeka kwekuzadza maorbitals kuburikidza nemufananidzo unotevera:
Iyi dhayaguru inotongwa neiyo Aufbau musimboti, iyo inopokana kuti maorbitals akazadzwa nekuwedzera kurongeka kwesimba, ndokuti, iyo orbital iyo ine mashoma simba ichazadza pakutanga.
Kuti uone kuti ndeipi orbital ine simba rakawanda, mashandiro n + l anoitwa. Kana iko kuvhiyiwa kwemaatomu maviri akasiyana kukaguma nenhamba yakafanana, uya ane nhamba n yakakwira anova nesimba rakawanda. Mune mamwe mazwi, kana paine tayi, iyo ine yakaderera nhamba n inozadzwa kutanga. Ngatizvionei nemuenzaniso:
4p: n + l -> 4 + 1 = 5
5s: n + l -> 5 + 0 = 5
Sezvo paine tie mu n + l mutemo, inozadza 4p pakutanga nekuti nhamba yayo n yakaderera.
Kernel Muenzaniso
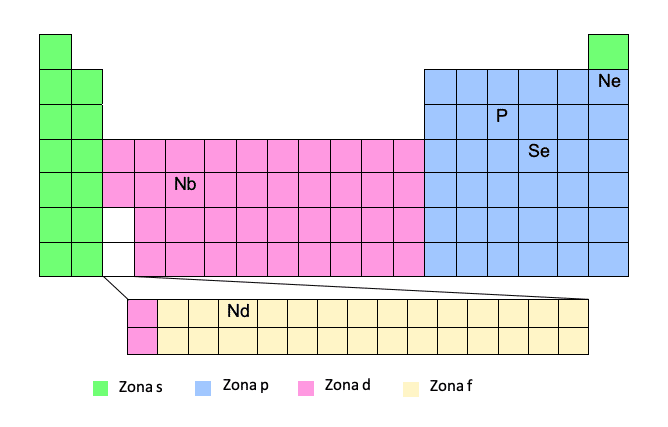
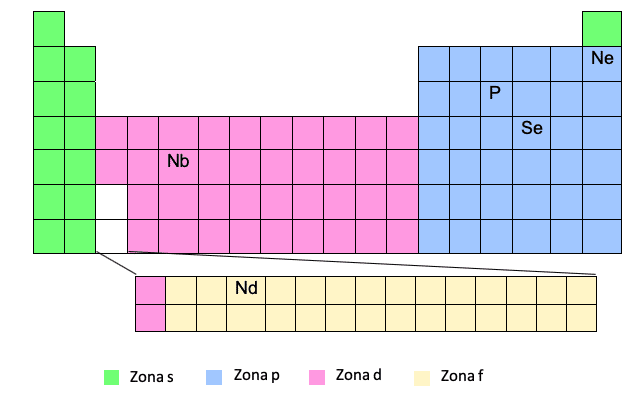
Kuti uwane kumisikidzwa kwemagetsi uchitevera iyi modhi unofanirwa kuziva tafura ye periodic zvakanyanya. Kana isu tine nhamba yeatomiki uye chinzvimbo chechinhu chiri mutafura, icho chidimbu chekeke!
Iyi nzira inoonekwa seyakareruka nzira nekuti inobvumidza kusave nekunyora yakakwana elektroni yekumisikidza. Nenzira iyi, tinogona kunyora zita rechinhu chakanakisa chegesi kumusoro mumabhureki, uyezve nzira kubva kune iro gasi rakanaka kuenda kuchinhu chiri kubvunzwa. Ngatione muenzaniso:Nekudaro, isu tinonyora iyo trajectory tichifunga iyo nhamba yenguva (mutsara wetafura ye periodic) uye "zone" uye, kana gadziriso yemagetsi yanyorwa, isu tinoburitsa huwandu hwehuwandu.
Iyo phosphor (P) ichanyorwa kubva kune yakapfuura gasi rakanaka, ndokuti, Neon:
P -> [Ne] 3s23p3
Ehe, iwe unofanirwa kungwarira neiyi nzira, sezvo nzvimbo d uye f inzvimbo dzakakosha. Sezvatinoita rwendo, munzvimbo d hatizoise iyo nhamba yenguva (mutsara), asi iyo nhamba yenguva kubvisa rimwe. Zvakafanana zvinoitika nenharaunda F, hatizoise nhamba yenguva, asi iyo nhamba yenguva kubvisa maviri. Iwe uchazvinzwisisa zvirinani nemimwe mienzaniso.
Nb -> [Kr] 5s14d4
Kunyangwe iri munguva yechishanu, kana tiri munharaunda d, tinobvisa 5.
Nd -> [Xe] 6s24f14
Kunyangwe iri munguva yechitanhatu, kana tiri munharaunda f, tinobvisa 6.
Kunze kwekugadziriswa kwemagetsi
Iyo elektroni yekumisikidza ine akati wandei akasarudzika maficha ayo, kana iwe usingazive nezvawo, anogona kukukonzera iwe hombe yekupa feeders. Asi usaparadzira kutya! Isu tichakuudza!
Nzvimbo F
Zone F inoonekwa pazasi petafura ye periodic, asi iri "yakamisikidzwa" mune mukaha watinoona wakachena, ndiko kuti, pakati pezvinhu zvekutanga nezvechipiri zvemitsara miviri yekupedzisira yenzvimbo D.
Unozviona here? Nechikonzero ichi, dzimwe nguva, patinofanira kunyora kumisikidzwa kwemagetsi kwechinhu munzvimbo F, semuenzaniso, Nd, tichafanirwa kuisa elektroni munzvimbo yeD yenhanho inoenderana nechinhu icho chiri mudunhu D icho chiri usati wapinda munzvimbo F.
Ce -> [Xe] 6s25d14f1
Boka 6 uye Boka 11
Boka 6 uye boka 11 shanduko yesimbi ine mana ne4 maerekitironi mumahombekombe avo ekupedzisira, zvichiteerana. Naizvozvo, kuve chinhu chakatsiga, iyo orbital inofara uye inorasikirwa neelectron, iyo inopfuura ichienda kune inotevera orbital, iyo d. Nenzira iyi, iyo orbital inosara ne electron; uye d na9, kana chiri chinhu cheboka 5, kana na6, kana chiri chinhu cheboka 10.
Heino muenzaniso:
Ag -> [Kr] mashanu24d9
Sezviri pachena, ichi chingave chemagetsi dhizaini yesirivha (Ag). Nekudaro, kurasikirwa neelectron kubva ku s orbital, zvinoita kunge seizvi:
Ag -> [Kr] mashanu14d10
Nekudaro, pane izvo zvinosiyiwa kumutemo uyu, senge Tungsten (boka 6), iyo inosara iine maerekitironi maviri mu s orbital uye mana mu d orbital.
Asi usazvidya moyo! Ivo vanowanzoitika (Cr, Cu, Ag naAu) vanoteedzera uyu mutemo.
Unoiwana here? Zvakanaka. Ndo zvese zvaunoda kuti uzive nezve electron kumisikidzwa. Ngatiendei kuhuwandu hwehuwandu!
Maitiro ekuwana huwandu hwehuwandu
Kuti tiwane huwandu hwehuwandu, tinofanirwa kuziva kuti mangani maerekitironi anokwana muhombodo imwe neimwe yekutenderera, tichifunga kuti maerekitironi maviri anokwana mumugwagwa.
- Rukoko s. Iyo ine orbital imwe chete, saka inogona kukwana maerekitironi maviri.
- Rukoko p. Iyo ine matatu maokitori, saka pane nzvimbo yematanhatu maerekitironi.
- Rukoko d. Iyo ine mashanu orbitals, saka inogona kubata gumi maerekitironi.
- Rukoko f. Iyo ine 7 orbitals, ndiko kuti, inobata gumi nematanhatu emagetsi.
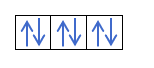
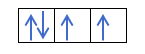
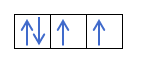
Iye zvino zvaunonzwisisa kuti maerekitironi maviri anokwana mumatanho ega ega, unofanirwa kuziva mutemo waHund. Mutemo uyu unoti kana uchizadza maokitori eiyo imwechete sublevel kana goko, semuenzaniso, p ganda, maerekitironi anozadza orbital munzira imwechete (yakanaka) uyezve mune imwe (isina kunaka). Iwe unoda kuzviona nemuenzaniso here?
Kana isu tine 2p4, ndiko kuti, iyo 2p inotenderera ine maerekitironi mana, haizare seizvi:
Ichazadza seizvi:
Uri kuzviwana here? Hukuru, ngatione maitiro ekuverenga iwo manhamba:
- Nhamba yenhamba n. Iyi nhamba inopindirana nenhamba yedanho rekupedzisira rekugadziriswa kwemagetsi. Semuenzaniso, kana iyo elektroni yekumisikidza ichiguma muna4s2, iyo nhamba yekutanga nhamba ichave ina.
- Nhamba yehuwandu l. Iyi nhamba inoenderana nerukoko rwekupedzisira rwazadzwa.
- Rukoko s -> l = 0
- Rukoko p -> l = 1
- Chidimbu d -> l = 2
- Rukoko f -> l = 3
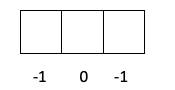
- Nhamba yeuwandu m. Iyo nhamba m inogona kuve chero kukosha pakati -l kusvika + l, saka zvinoenderana neiyo sublevel mune iyo kusiyanisa elektroni, ndiko kuti, kana iri s, p, d kana f. Maitiro ekuverenga nhamba iyi zvakati omei, ngatizvionei nemadhirowa mashoma.
- Layer s -> Sezvataona, iyo l yakakosha 0, saka iyo m inogona chete kukosha 0.
- Layer p -> Iyo l yakakosha 1, saka m inogona kuva -1, 0 kana 1.
- Rukoko d -> Iyo l iri 2, saka iyo m inogona kuve -2, -1, 0, 1 uye 2.
- Rukoko f -> Iyo l yakakosha 3, saka m inogona kuva -3, -2, -1, 0, 1, 2 na3.
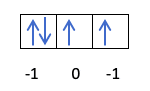
Iwe unotoziva kuti maokitoreti anozadzwa sei, saka iyo huwandu huwandu m huchave nehunokosha hwegomba pane yekupedzisira yakadhonzwa elektroni iri. Unoyeuka uyu muenzaniso kubva kare?:
Mune ino kesi, iyo m ichave -1, sezvo iri p ganda (matatu maokitobhu), kana paine mana maerekitironi, yekupedzisira yekuzadza ingave yakaipa yekutanga orbital.
- Nhamba yeuwandu s. Huwandu hwehuwandu hunogona kungokosha ½ uye -½. Kana iyo elektroni yekupedzisira yakadhonzwa iri yakanaka, ndokuti, museve wakwira, iyo s ichave ½. Kune rimwe divi, kana elektroni rekupedzisira rakazadza orbital risina kunaka, ndiko kuti, nemuseve unonongedza pasi, iyo s ichave -½.
Exercises uye mienzaniso
Hongu, isu tatoziva kuti zvese izvi ruzivo rwakawanda, asi iwe uchazvinzwisisa zvirinani nemimwe mienzaniso. Apa toenda!
Muenzaniso 1
Selenium (Se) -> Atomic nhamba: 34
- Isu tinonyora iyo yemagetsi gadziriso. Tiri kunyora kumisikidzwa kwema electron zvinoenderana neMoeller dhayagiramu, tichifunga kuti iyo s, p, d uye f orbitals ine 2, 6, 10 uye 14 maerekitironi zvichiteerana. Tiri kunyora kumisikidzwa nekuwedzera iyo nhamba yemagetsi, iyo yakanyorwa seyakajekesa.
1s22s22p63s23p64s23d104p4
Sezvo iyo 4p orbital isingazadze, sezvo maerekitironi aigona kuwedzera kusvika makumi matatu neshanu, isu hatiise 36p6asi 4p4.
- Isu tinoburitsa huwandu hwehuwandu. Kuti tiite izvi, isu tinotarisa iyo valence kana kusiyanisa elektroni, ndokuti, iyo yekupedzisira elektroni iyo izere iyo orbital. Mune ino kesi, isu tichatarisa 4p4.
- Yakakura nhamba yehuwandu. Chikamu chekupedzisira chesimba chekuzadza chaive 4.
n = 4
- Sekondari huwandu huwandu. Simba rekupedzisira sublevel yekuzadza yaive p orbital.
l = 1
- Nhamba yemagineti yakawanda. Kana isu tiri kudhirowa maerekitironi, yekupedzisira kuzadza ichave yekutanga orbital yeiyo p ganda.
m = -1
- Spin yakawanda nhamba. Electron yekupedzisira kutora p orbital ine museve wezasi.
s = -½
Muenzaniso 2
Ndarama (Au) -> [Xe] 6s14f145d10
- Yakakura nhamba yehuwandu -> n = 5
- Sekondari huwandu huwandu -> l = 2
- Nhamba yemagineti yakawanda -> m = 2
- Spin yakawanda nhamba -> s = -½
Uye ndizvozvo! Ikozvino yave nguva yako, unogona here kuita iyo elektroni yekugadzirisa uye uwane huwandu hwehuwandu hwezvinhu zvinotevera?
VaCr (24), Rb (37), Br (35), Lu (71), Au (79)